Frequently Asked Questions
As a patient, family member or patient advocate, you are bound to have questions. Below are answers to frequently asked questions about clinical research studies, xerostomia, and the AQUAx2 study.
Clinical research studies help doctors and scientists determine whether a medical strategy, drug, or device is safe and effective for humans. Participation in clinical research studies is voluntary, and participants may decide to stop participating at any time without that decision affecting their medical care.
Gene therapy/ gene transfer is the process of placing new genetic material, such as AAV2-hAQP1, into a person. The investigational gene therapy in this study uses a virus called adeno-associated virus (AAV) as a delivery vehicle to transport the human Aquaporin-1 (hAQP1) gene into the parotid salivary glands and to cause this hAQP1 gene to be made in the glands.
The parotid glands are a pair of salivary glands located under the skin in front of each ear and extending along the angle of the jaw. Radiation-induced damage to these glands reduces the amount of saliva they make.
You will be randomly assigned to one of three treatment groups via a process called randomization (decided by chance). Two of these three groups will receive active gene therapy (one of two different strengths of the study drug) and the third will receive a placebo (an inactive solution that does not contain the study drug).
A placebo is an inactive substance or treatment that looks the same and is given in the same way as the study drug. You have a 1 in 3 chance of receiving a placebo in this trial.
Participants who receive placebo in the AQUAx2 study will be offered the study drug in the follow-up study, provided they continue to qualify for treatment.
The procedure for administering the study drug takes place in a clinical setting such as physician’s office or dentist’s chair. The participant opens their mouth and the investigator then inserts a very small catheter into each parotid salivary gland through a small opening in the cheek next to the upper second molar. A syringe is then attached to all the study drug to be infused into the glands. The procedure takes about 15 minutes per side. No anesthesia is needed.
To alleviate travel and geographic hurdles, we have selected sites across the US, Canada and the UK.
We have a travel support team that will help coordinate travel and accommodations and assist with reimbursement for study-related travel.
In the US & Canada, you will be given a stipend for incidental expenses for travel within 50 miles of the study site. If you need to travel more than 50 miles from your home to the study site, you will be reimbursed for all travel-related costs.
In the UK, travel will be arranged by the hospital and reimbursed by the Sponsor.
Clinical trial participants may withdraw from a study at any time without that decision affecting the medical care to which they are otherwise entitled. Before deciding to take part in a clinical study, potential participants should thoroughly review the study informed consent form and discuss any questions they have with the investigator.
No. All study-related procedures and evaluations are paid for by the sponsor. Participants do not need medical insurance and any insurance you do have will not be billed.
Known and potential risks of the study will be explained to you by your doctor before you decide to participate. If you choose to join the study, your health will be closely monitored by your medical team.
In the Phase 1 study, the study drug was found to be safe and was well-tolerated by all participants.
The study consists of a one-time treatment of study drug. After the treatment is given, the study visits will be conducted to monitor safety and changes in symptoms and saliva flow rate over time, as well as to monitor general health.
Data from the Phase 1 study are available at the Investors & Media Page at MeiraGTx.com.
Geographical Study Locations
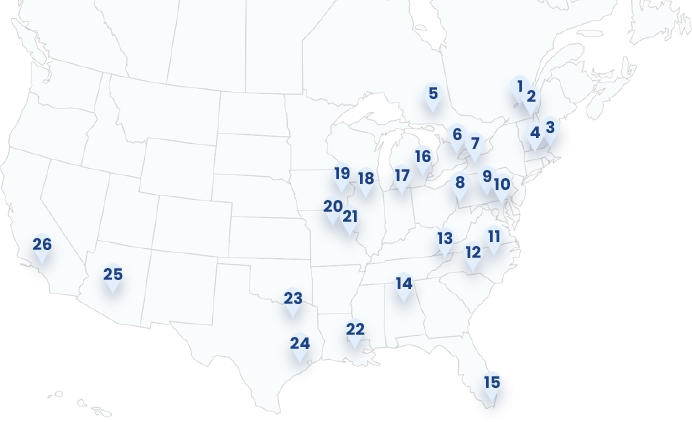
1. Trois-Rivières (Trois-Rivières, CAN)
2. U de Sherbrooke (Sherbrooke, CAN)
3. Brigham (Boston, MA)
4. Tufts (Boston, MA)
5. Health Sciences North (Sudbury, CAN)
6. UHN Princess Margaret (Toronto, CAN)
7. Erie County Medical Center (Buffalo, NY)
8. Allegheny (Pittsburgh, PA)
9. Penn State (Hershey, PA)
10. Johns Hopkins (Baltimore, MD)
11. UNC Chapel Hill (Chapel Hill, NC)
12. Atrium Health (Charlotte, NC)
13. Ballad Health (Johnson City, TN)
14. University of Alabama (Birmingham, AL)
15. Baptist Health (Miami, FL)
16. Henry Ford (Detroit, MI)
17. Goshen Center (Goshen, IN)
18. Northwestern (Chicago, IL)
19. University of Iowa (Iowa City, IA)
20. University of Missouri (Columbia, MO)
21. Wash U (St Louis, MO)
22. OLOL (Baton Rouge, LA)
23. University of Texas (Dallas, TX)
24. Houston Methodist (Houston, TX)
25. Banner Health (Phoenix, AZ)
26. City of Hope (Duarte, CA)
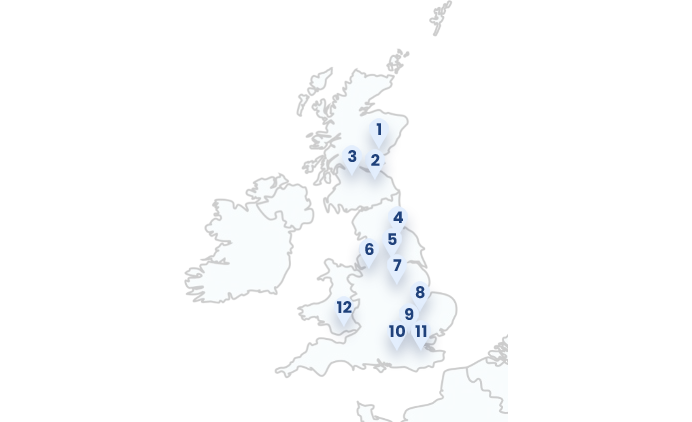
1: Ninewells Hospital (Dundee, Scotland)
2: Western General Hospital (Edinburgh, Scotland)
3: Glasgow Royal Infirmary (Glasgow, Scotland)
4: York & Scarborough Hospital (York, England)
5: Leeds Dental Institute (Leeds, England)
6: Manchester Royal Infirmary (Manchester, England)
7: Nottingham University Hospital (Nottingham, England)
8: Addenbrooke’s Hospital (Cambridge, England)
9: University College London Hospitals (London, England)
10: Guys & St. Thomas (London, England)
11: Royal Marsden (London, England)
12: Cardiff University Hospital (Cardiff, Wales)

More Information

SPOHNC is dedicated to raising awareness and meeting the needs of oral and head and neck cancer patients through its resources and publications.

The Swallows Head & Neck Cancer Support Group supports all people affected by head and neck cancers, patients, caregivers, friends, or relatives. It is our intention for every person affected by head and neck cancer to have access to support at the point they need it, by the method of their choice, on a 24/7 basis.

The THANC (Thyroid, Head & Neck Cancer) Foundation develops research and education in the early detection and treatment of thyroid, head and neck cancer—supporting advancements in new therapeutic approaches and the alleviation of suffering and functional impairment that patients experience along their journey.

The THANC Guide strives to provide information that is written by experts but easy to understand, for adults, teens and children, on diagnosis, treatments, emotional challenges, medical decision making and much more.

The Head and Neck Cancer Alliance’s mission is to advance prevention, detection, treatment and rehabilitation of oral, head and neck cancer through public awareness, research, advocacy and survivorship.

The NFOSD aims to enhance direct patient support, education, research and raising public, professional and governmental awareness. Their mission is to advance the prevention and treatment of swallowing disorders in our lifetime.
Visit our Frequently Asked Questions page
If you have more questions or would like to explore online resources with useful information, visit our Resources page.
